How to heal skin without a scar
More than 100 million new skin scars appear annually and scars themselves often cause additional mental and physical injury (e.g., burn victims, scars over joints). Despite a multitude of products, none have demonstrated effective scar reduction or prevention. We aim to heal injured skin without a scar.
Salamanders regrow tissues, organs, and even whole body parts. Some of this ability is still retained in humans! Human livers regenerate, and digit tip amputations in children heal without a scar. These examples suggest that scarless wound healing, though rarely active, still exist in humans. We need to figure out ways to re-engage these pathways.
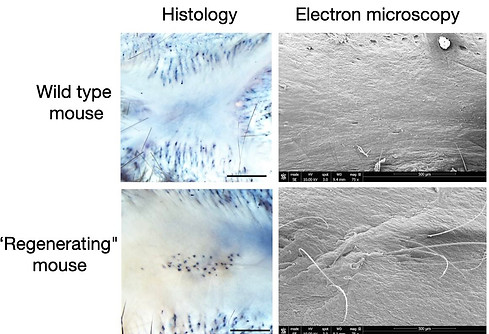
Our group and others have established injury models in mice that permit scarless wound healing. For example, punching small holes in the ear typically causes the skin to heal and the hole to remain (similar to a human ear-ring hole, left figure). We have discovered some strains of mice where the holes close and heal completely without a scar. The healed tissue contains regenerated cartilage and hair follicles. Scanning electron microscopy illustrates the regenerated unpigmented hairs (see right figure).

Over the past 10 years, we have used these mice in different experiments, including parabiosis where we surgically connect two living mice together (see video below). These efforts identified multiple molecular pathways involving skin, immune cells, and sensory nerves that regulate the switch between scar and scarless wound healing (Genes and Development, 2015, Cell Reports, 2018, Science Immunology, 2020, Cell Reports, 2024). We have increased the frequency of complete ear hole closure in a mouse from 1% to 90%. We are eager to test these pathways in human skin. Taken together, we aim to develop novel therapies to promote scarless skin regeneration in humans.
Personalized medicine and human skin diseases